
Biological Treatment of Wastewater
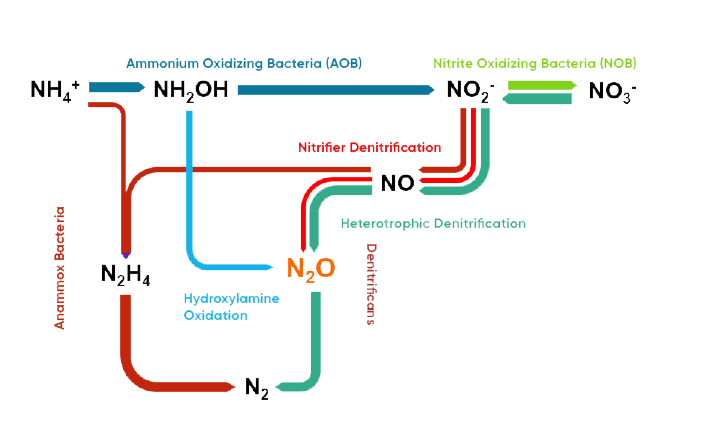
N2O Occurs in both Denitrification and Nitrification
During wastewater treatment, the biological conversion of ammonium (NH4+) to free nitrogen (N2) forms N2O.
Originally, scientists described N2O as an intermediate product of the denitrification, the conversion of nitrate (NO3-)/nitrite (NO2-) to free nitrogen. However, today the scientific community has documented that N2O is a significant byproduct from the nitrification as well. The conversion of NH4+ to NO2- and to NO3- results in the formation of N2O.
You can read about the main processes and factors for nitrous oxide formation below. In addition, you can find a schematic overview from the Global Water Research Coalitions state of science report “N2O and CH4 Emission from Wastewater Collection and Treatment Systems”.
The Sources of N2O Formation
Nitrification is the stepwise oxidation of NH4+ to NO2- and to NO3- with the use of O2 as the electron acceptor. The predominant bacteria responsible for the conversion from NH4+ to NO2- are Ammonium Oxidizing Bacteria (AOB) and Nitrite Oxidizing Bacteria (NOB).
Denitrification is the stepwise reduction of NO3- to NO2- and further on to nitrogen (N2) via NO and N2O intermediates and with the use of a carbon source and the electron donor. The predominant bacteria responsible for the conversion are Heterotrophic Denitrifying Bacteria (HET).
The Nitrifier Denitrification Pathway
Ammonium Oxidizing Bacteria (AOB) can under oxygen limiting conditions reduce NO2- to N2O, but not all the way to free N2. This pathway is called the Nitrifier Denitrification pathway. Actually, it is believed to be one of the major sources to N2O formation and emission. Energy optimization of the aeration strategies especially stimulate this process.
N2O as the End-product
Furthermore, N2O can also be the end-product during denitrification if there are trace levels of oxygen, as oxygen inhibits the reduction process of N2O to N2. Finally, hydroxylamine oxidation can also result in N2O formation via intermediates of the biological hydroxylamine oxidation or chemically.
Write us a message. We strive to answer within one workday.
If we are online and available to chat, you can also click the blue icon to the right.